Reducing nitroarenes to amines without need for extreme conditions or toxic reagent production
A team of researchers affiliated with entities in the Czech Republic, Greece and Germany has developed a way to reduce nitroarenes to amines that does not produce toxic reagents and does not involve extreme conditions. They've published their results in Nature Nanotechnology.
Reducing nitroarenes to amines is a common procedure in commercial applications—it is part of the process involved in creating such products as polymers, plastics and paint. The current reduction method requires processing at temperatures as high as 100 degrees Celsius, the use of noble metal catalysts, and hydrogen gas under high pressure. Such conditions have led scientists to look for other ways to get the job done. One promising approach involves the use of plasmonic interactions. In this new effort, the researchers expand on this research.
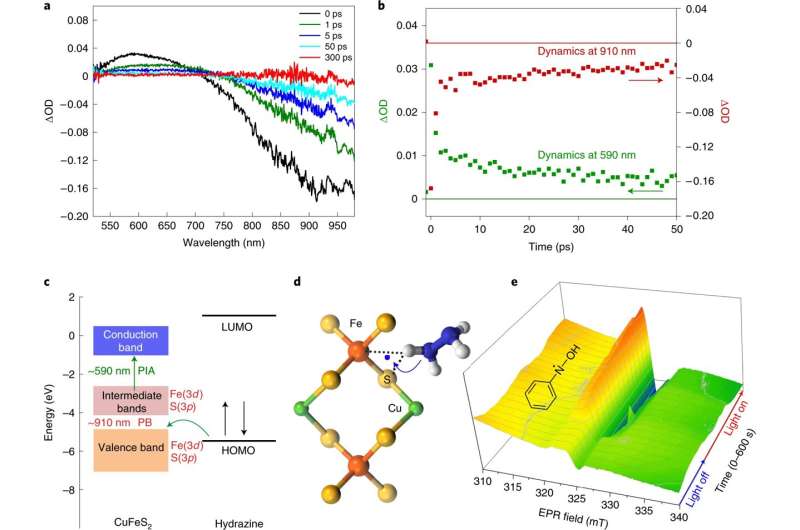
The reduction process they developed starts with chalcopyrite nanocrystals with plasmon resonance similar to gold nanoparticles. The nanocrystals are not only less expensive, the researchers note, but they also have improved catalytic properties. The result is an increase in electron-hole pairs. In their process, the reactants are absorbed by the nanocrystals.
Next, the researchers added the crystals to a hydrazine and nitrobenzene solution and then bombarded the results with blue light for two hours. The hydrazine reduced the nitrobenzene to aniline with a 100% yield. The researchers also note that the process was carried out at room temperature, though the reaction did increase the temperature of the solution from 25 degrees to 58 degrees Celsius, which sped up the reaction. It also does not produce toxic reagents. And finally, it involves the use of copper iron sulfide, which is easily obtainable.
The researchers note that their process delivered turnover frequencies that were unattainable in other reactions and that it has an order of magnitude reduced cost-normalized rate for selectively reducing nitroarenes.